BiomX logo (BiomX website)
BiomX Inc. (NYSE:PHGE) is a clinical-stage biotech company that develops bacteriophage (“phage”) cocktails to treat various diseases utilizing its next-generation BOLT (BacteriOphage Lead to Therapy) phage therapy and synthetic biology XMarker platforms.
Phages are viruses that are natural predators of bacteria that are specific to particular bacterial strains. (Source: BiomX’s website). There has been increased interest in phage therapies, which have been known for many years to be safe to administer to patients, as a result of the rapid increase in antimicrobial resistance, resulting in multi-drug resistant and pan-drug resistant bacterial infections.
Cystic Fibrosis is caused by a mutation on the CFTR gene. 80% of deaths are from respiratory failure. (Source: BiomX website). Many Cystic Fibrosis patients suffer from debilitating chronic lung infections caused by certain strains of bacteria, most commonly Pseudomonas aeruginosa bacteria (“PsA” or “P.aeuginosa”). CF patients suffering from such chronic infections have become resistant to standard of care antibiotics which has led to increased hospitalizations and death.
This has created a large unmet need for these CF patients suffering from chronic lung infections caused by PsA who no longer respond to standard of care antibiotics. BiomX BX004 phage cocktail therapy, if ultimately shown to work, could provide a solution.
Investment Thesis
BiomX is currently a binary high-risk, high-reward investment. After announcing better-than-expected Part 1 BX004 clinical trial results in February of this year, BiomX is about to release top-line results from Part 2 of its Phase 1b/2a placebo-controlled, clinical study of BX004 phage cocktail in treating chronic lung infections caused by PsA in 24 Cystic Fibrosis patients. (Source: BiomX’s November 14, 2023 press release).
With Fast Track Designation, great unmet need, and a potential US market for BX004 in CF chronic lung infection caused by PsA of over $1 billion, if Part 2 of its Phase 1b/2a clinical results are successful, BiomX share price could significantly increase. On the other hand, if the Part 2 trial results are poor, BiomX’s share price will likely plummet. It could quickly get into financial trouble if these trial results are poor. BiomX’s current cash position is sufficient to cover ongoing costs to Q3 2024.
For the reasons set out in this article, I’m cautiously optimistic about the upcoming Part 2 trial results. No guarantees of course.
BX004 FDA Fast Track Designation: In its August 9, 2023 press release providing a corporate and Q2 2023 financial update, BiomX announced that BX004 received FDA Fast Track designation,
…for the treatment of chronic respiratory infections caused by Pseudomonas aeruginosa (PSA) bacterial strains in patients with CF. The FDA’s Fast Track designation is a process designed to facilitate the development and expedite the review of drugs to treat serious conditions and address significant unmet medical needs. The FDA defines addressing a significant unmet medical need as providing a therapy where none exists or providing a therapy which may be potentially better than available therapies. The benefits of Fast Track designation include but are not limited to early and frequent communication with the FDA throughout the entire drug development and review process. In addition, a drug with Fast Track designation is eligible for rolling submission and priority review of its Biologics License Application and/or New Drug Application….”
ClinicalTrials.Gov NCT05010577: For further reference regarding BiomX’s BX004 Phase 1b/2a, Parts 1 and 2, clinical trial design, the identity of its 28 clinical sites and other relevant information see clinicaltrials.gov NCT05010577. There were 28 clinical sites listed, including 18 sites in the U.S., five in Israel, two in the Netherlands, two in Spain, and one in Czechoslovakia.
Reasons for Optimism: While BX004 clinical results to date have been in a small number of patients, with limited treatments, and for a short duration, I am cautiously optimistic about the upcoming BX004 Part 2 study results for a number of reasons.
Compassionate Use Phage treatments for CF patients with chronic lung disease due to PsA (BiomX November 14, 2032, corporate presentation)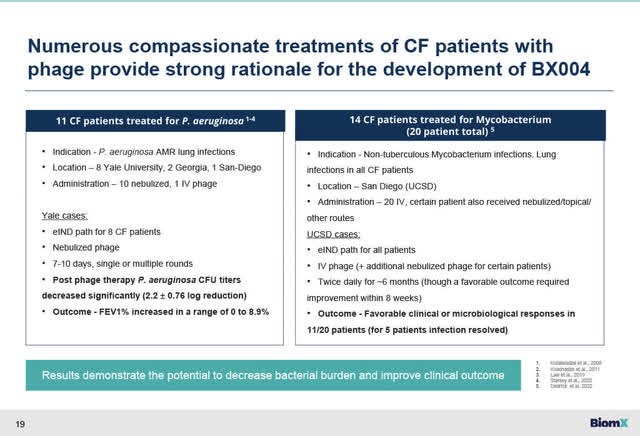
- encouraging clinical results using phage treatment in treating CF patients have been reported over the past number of years summarized in the slide above;
- Recently clinical results posted on January 24, 2023 on MedRxiv (preprint service for Health Sciences) by Yale University (Cystic Fibrosis program) summarizing the clinical results of 9 CF patients with chronic lung infections caused by PsA (no longer responsive to standard of care antibiotics because of drug resistance). These 9 CF patients were treated with phage treatments for 7 to 10 days with either tailored phage cocktails (in 6 patients) or a single phage (3 patients) formulation, in a nebulized treatment format under a FDA compassionate use program. The clinical results for these 9 CF patients showed safety and effectiveness in reducing PsA levels and improving lung function in FEV1 measurements (p<.004). The Yale study was in part funded by the Cystic Fibrosis Foundation and the NIH. While this was not a placebo-controlled study and only in 9 patients, the clinical results are nonetheless encouraging; the results showed that phage therapies are potentially effective in treating this type of chronic lung infection caused PsA, reducing bacterial infections and improving lung function. There was a more significant FEV1 improvement in those CF patients who started with an expected FEV1 >40%. This is significant as BiomX’s Part 2 study only recruits patients whose FEV1 is >40%, which is already a reasonably severely compromised lung function. See the link to the Yale study 9 CF patient results last published in January 2023; and
- BiomX’s strong clinical results from its 9-patient, double-blind, placebo-controlled, Part 1 of the clinical study released in February, as discussed below. This was also a small group of only 9 patients (7 in the treatment group and 2 treated with a placebo) who were effectively only given 4 days of full BX004 treatment. BiomX is also funded, in part, by the Cystic Fibrosis Foundation which also provided input into BiomX’s trial design for its BX004 CF studies. The Phase 1b/2a clinical trial is the first reported double-blind placebo-controlled study evaluating a cocktail-based phage product to demonstrate notable reductions in bacterial burden in cystic fibrosis.
Part 2 of the BX004 Phase 1b/2a study was designed to enroll 24 patients, in a double-blind, placebo-controlled study, (with 16 patients in the treatment group and 8 patients in the placebo group) multi-center study conducted in the U.S., Israel and Europe. According to BiomX’s August 9, 2023, press release Part 2 patient enrollment is expected to exceed the initial 24-patient estimate although no precise numbers have been provided.
On October 4, 2023, BiomX announced the completion of patient dosing in Part 2 of the Phase 1b/2a study.
I last wrote about BiomX on Seeking Alpha more than 2 years ago when I published an article about BiomX on October 6, 2021, BiomX: Undervalued With Multiple Upcoming Catalysts.
Restructuring of BiomX in May 2022: BiomX announced on May 2, 2022, that it was restructuring. By that time BiomX’s share price had collapsed. As part of the restructuring BiomX announced cutting 50% of its staff, putting its various ongoing programs on hold, and focusing almost all of its efforts on advancing only its BX004 phage drug candidate in its Phase 1b/Phase 2a program to treat chronic pulmonary infections caused by P. aeruginosa in patients with Cystic Fibrosis.
If the upcoming BX004 Part 2 Phase 1b/2a trial results are successful, this strategy may pay off. We will find out in the coming days.
BiomX Corporate Summary
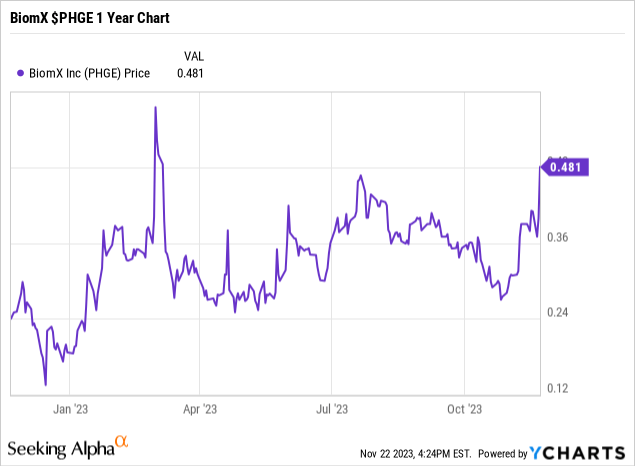
Symbol: PHGE trading on both the NYSE and on the Tel Aviv Stock Exchange
Common shares outstanding: 45,979,730 as of September 30, 2023 (including 2,776,428 pre-funded warrants issued February 2023 and 11,834,286 pre-funded warrants issued April 2023)
52-week Share price range: $0.132 to $0.69
BiomX share price; close of trading November 22, 2023: $0.45
Market cap (based upon 45,979,730 common shares including 14,610,714 pre-funded warrants): $20.7 million
Cash and equivalents as of September 30, 2023: $23.4 million (Source: November 14, 2023 press release)
Long-term debt: $12,070,000 as of September 30, 2023, bearing interest at prime plus 5.70%, requires $5 million cash to be held by BiomX (Source: Form 10-Q filed with the SEC filed November 14, 2023)
Cash sufficient to fund operations to Q3 2024 (Form 10-Q filed November 14, 2023)
Major institutional shareholders include Orbimed, Johnson & Johnson, Cystic Fibrosis Foundation, and Takeda (Source: Bloomberg search November 22, 2023)
What is BX004?
BX004 itself is BiomX’s proprietary phage cocktail, composed of between 3 to 5 selected phages with different functions (in a nebulized delivery format) targeting CF patients with chronic PsA lung infections. The exact number and details of the phages in BX004 are not publicly disclosed.
BX004 – Nebulized delivery format of phage cocktail with potential to treat CF patients with chronic PsA lung infections (BiomX Feb 23, 2023 presentation)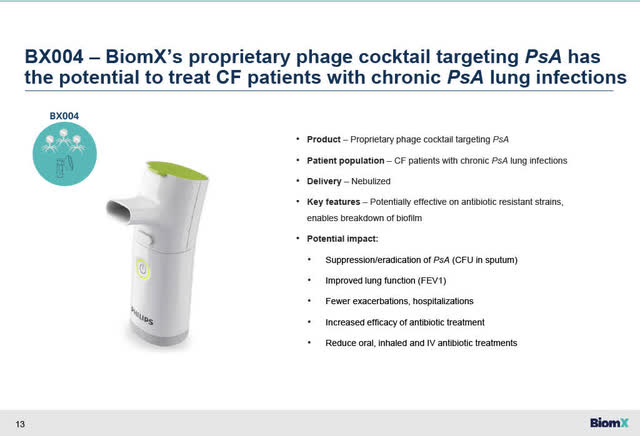
BX004: Part 1, Phase 1b/Phase 2 Clinical Trial Results in treating chronic pulmonary infections in Cystic Fibrosis
The data from Part 1, Phase 1b/Phase 2a clinical trial achieved not only its primary goal to assess the safety and tolerability of BX004 but also showed preliminary evidence of efficacy, despite those patients only being treated with the full twice daily dose of BX004 for 4 days. During the first 3 days of the trial, patients were administered a nebulized placebo dose; on the second day, patients were administered a single low dose, and on the third day, with a single high dose of BX004.
BiomX’s CEO Jonathan Soloman has described these early Part 1 clinical results as surprisingly good and exceeding his expectations particularly as the patients were effectively only given 4 days of BX004 treatment at full twice daily dosing.
The Part 1 BX004 results showed a -1.42 log PsA reduction in the treatment group (N=7) at Day 15 (compared to Baseline) compared to a -0.28 log in the placebo group (N=2). A -1.0 log represents a 90% reduction while a -2.0 log reduction is a 99% reduction.
BX004 Part 1 Ph 1b/2a results (BiomXNovember 14 2023 corporate presentation)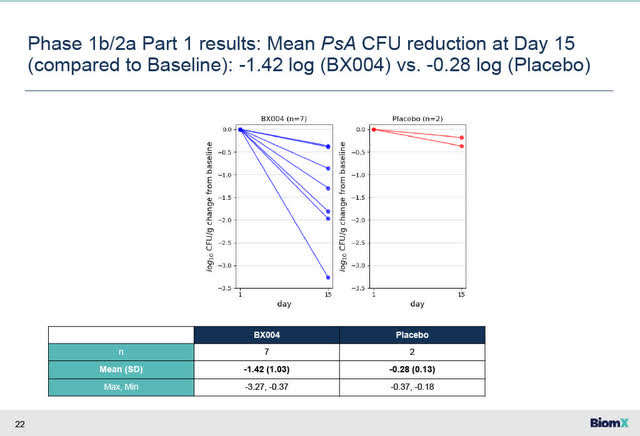
The following table (source: Wikipedia) summarizes Log reductions
| Log reduction | Percentage |
|---|---|
| 1-log reduction | 90% |
| 2-log reduction | 99% |
| 3-log reduction | 99.9% |
| 4-log reduction | 99.99% |
| 5-log reduction | 99.999% |
While Part 1 of this Phase 1b/2a trial had only 9 patients (7 being treated with BX004 with standard of care antibiotics, and 2 being treated with a placebo along with standard of care antibiotics) and being a relatively short duration of treatment, the results were very encouraging.
Chronic PsA Infections in CF Patients
By way of background, for many CF patients, chronic PsA infections are an ongoing serious problem that has become resistant to standard-of-care antibiotics and have become a driving force in morbidity and deaths in CF patients.
Chronic PsA infections are a persistent problem due to antibiotic resistance driving morbidity and mortality in CF (BiomX Feb 23, 2023 presentation)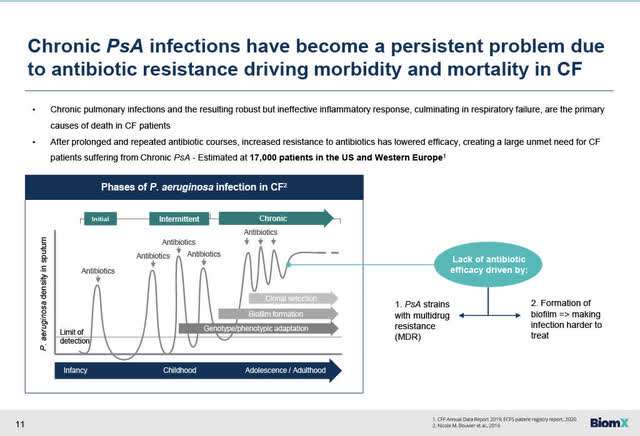
On Day 15 after the commencement of treatment in Part 1 BX004 trial, the mean P. aeruginosa bacterial burden in the lung of these CF patients was reduced by 1.42 log10 CFU/g compared to 0.28 log10 CFU/g in those receiving placebo.
Cystic Fibrosis – >80% deaths from respiratory failure (BiomX February 22, 2023 Corporate Presentation)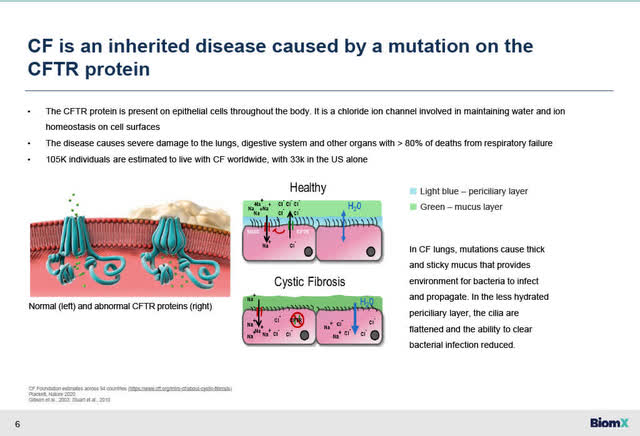
Chronic PsA infections in CF patients are a serious problem because of antibiotic resistance. PsA strains with multi-drug resistance are caused in part because of the formation of biofilms which makes infection even harder to treat.
The unmet need is that of the estimated 14,600 CF patients in the U.S., about 65% (approximately 8,000 CF patients) are not being successfully treated with antibiotics when they have a lung infection caused by PsA. These lung infections can be serious and can lead to hospitalization or even death.
BX004 was shown in pre-clinical studies to penetrate the biofilm and show activity even on antibiotic-resistant strains.
Furthermore, in other studies involving phage therapies in treating CF patients suffering from PsA strain lung infections resistant to antibiotics, there were compassionate use authorized treatments that were successful in treating a limited number of patients.
Pseudomonas Aeruginosa bacteria associated with decreased lung function and damage in CF patients (BiomX February 22, 2023 corporate presentation)
BX004 Market Opportunity
BiomX estimates in its November 14th corporate presentation, see slide below, that there are approximately 8,000 CF patients in the U.S. who suffer from chronic lung infections due to PsA infections who are not responding well to standard of care antibiotics. Assuming potential pricing of $100,000 to $120,000 per year for BX004, if approved by the FDA, BiomX estimates that BX004 has a potential market opportunity in this indication alone of greater than $1 billion opportunity in the U.S. alone, and a $1.6 billion opportunity including markets outside the U.S.
BX004 Market Opportunity in CF (BiomX November 14 2023 corporate presentation)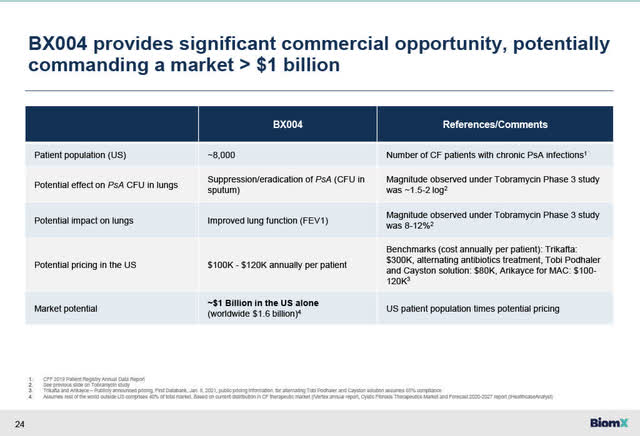
Regulatory Pathway for BX004
Regulatory Pathway for BX004 (BiomX February 22, 2023 corporate presentation)
Analyst Coverage and Price Targets
Ladenburg Thalmann (Michael Higgin) Price target $4.00, Nov. 15 2023
HC Wainwright (Dr. Joseph Pantginis), Price target $6.00, Nov. 14, 2023
Risks:
BiomX is an early-stage biotech company advancing its BX004 phage cocktail therapeutic in CF chronic lung infection. If the current Part 2 Phase 1b/2a BX004 trial is unsuccessful or its results otherwise poor, the BiomX share price could collapse and investors could lose most or even all of their investment. In addition, even if the trial results are successful, BiomX will need to raise additional funds next year to continue its clinical trial programs which could be dilutive, and there will still be a requirement for at least one Phase 3 trial with all of its accompanying costs and risks.
For further information about the potential risks, see BiomX’s Form 10-Q filed with the SEC.
This is a high-risk, high-reward investment suitable for investors with a high risk tolerance.
Investment Conclusion:
For the reasons set out above, while I am mindful that BiomX is a high-risk investment at this stage, I’m cautiously optimistic about the outcome of Part 2 of the BX004 Phase 1b/2a study in CF patients with chronic lung infections due to PsA infections. A positive trial outcome could lead to a major increase in BiomX’s share price (closed at $0.45 on November 22, 2023).
With a market cap of approximately $20 million, and long-term debt of approximately $12 million, the upcoming trial results create a binary outcome for BiomX. While successful Part 2 results could lead to a major win for investors, a poor outcome to lead to BiomX share price collapse and financial loss as the company only has cash to cover its costs to Q3 2024.
The Part 2 trial results will be released any day. We’ll find out BiomX’s fate very soon.
Editor’s Note: This article covers one or more microcap stocks. Please be aware of the risks associated with these stocks.
Read the full article here