Our wretched species is so made that those who walk on the well-trodden path always throw stones at those who are showing a new road.”― Voltaire.
Today, we put Innoviva, Inc. (NASDAQ:INVA) in the spotlight. As can be seen in the chart below, it has been somewhat of a rollercoaster ride for the shareholder over the past half-decade. The stock appears cheap on a trailing earnings basis, and a beneficial added some significant shares to their stake in the second quarter of this year. The company also named a new CFO late in August. This followed the appointment of a new CEO in July. An analysis follows below.
Seeking Alpha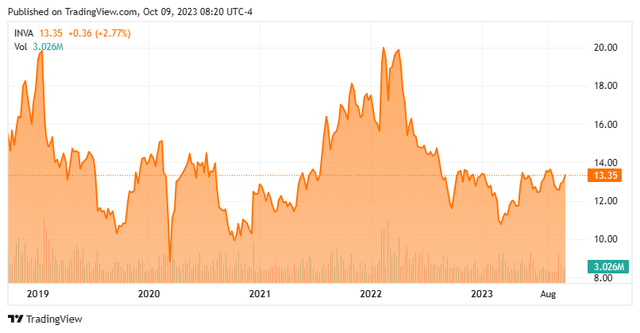
Company Overview:
Innoviva, Inc. is a small biopharma concern headquartered just outside of San Francisco in Burlingame, CA. The company is focused on the development and commercialization of pharmaceutical products. The stock currently trades just above $13.00 a share and sports an approximate market capitalization of $870 million.
Product Portfolio (Company Website)
Innoviva, Inc. acts as a diversified holding company with a portfolio of royalties and other healthcare assets. The main asset Innoviva currently possesses is the royalty revenue from several products it gets from drug giant GSK plc (GSK). These include products like RELVAR®/BREO® ELLIPTA® and ANORO® ELLIPTA®. The company sold its subsidiary Theravance Respiratory Company as well as its TRELEGY® ELLIPTA® royalty stream in July 2022. This asset was jointly owned by Theravance Biopharma (TBPH) at the time. This was sold to Royalty Pharma (RPRX) in the summer of 2022 for $1.31 billion upfront, as well as up to $300 million in milestone payments. It should be noted that Theravance Respiratory Company only had a 15% stake in this joint venture.
The company also gets net product sales and license revenue from homegrown products like XERAVA®, GIAPREZA® and recently approved XACDURO®, which became the first pathogen-targeted therapy green-lighted to treat hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. The approval was garnered by Entasis Therapeutics, an affiliate of Innoviva Specialty Therapeutics, which is a unit of Innoviva.
Net sales and license revenue for the company’s non-Glaxo connected products came in at $18.7 million. This included a $3 million milestone payout from Innoviva’s partner for the FDA approval of XACDURO®. GIAPREZA® net sales contributed $11.2 million during the quarter, and XERAVA® net sales were $4.5 million.
Second Quarter Results:
Innoviva, Inc. reported second quarter numbers on August 2nd. Innoviva had a GAAP profit of 2 cents a share as revenues fell just over 25% on a year-over-year basis to $81 million. This was the result of the loss of the TRELEGY® ELLIPTA® royalty stream by the sale previously noted. Overall sales were also some $13 million over expectations for the quarter.
RELVAR®/BREO® ELLIPTA® contributed $54.4 million in royalties for the quarter, compared to $111.7 million in 2Q2022 when they also included TRELEGY®. ANORO® ELLIPTA® royalties rose $1.7 million to $11.3 million.
Analyst Commentary & Balance Sheet:
There has not been any new analyst firm commentary on the stock since May of this year. In March through May of this year, Goldman Sachs ($13 price target) reissued its Hold rating and Morgan Stanley ($10 price target) maintained its Sell rating. On May 10th, EF Hutton ($22.50 price target) reiterated the only Buy rating on the stock currently.
Approximately one out of every six shares outstanding is currently held short. The CEO added just over $32,000 to his stake in the firm in March of this year, while a beneficial owner purchased over $9 million worth of shares in total in May and June. That has been the only insider activity in the stock so far in 2023.
The company ended the second quarter with just over $170 million of cash and marketable securities on its balance sheet. It also had $81 million worth of sales and milestone receivables on its books at the end of the first half of the year. Based on perusing its balance sheet, the company has some $385 million in long-term debt. The company repurchased some $9.2 million of its own stock in the second quarter.
In July, after the quarter closed, Innoviva entered into a credit and security agreement with Armata Pharmaceuticals, Inc. (ARMP). The company also invested a total of $25 million to advance Armata’s pipeline of therapeutic phage candidates. This will also support the build-out of Armata’s state-of-the art cGMP manufacturing facility. These efforts are targeting infections caused by the likes of Pseudomonas aeruginosa and Staphylococcus aureus. A director of Innoviva will be Armata’s new CEO as well.
Verdict:
The company made $2.37 a share in FY2022 on just over $330 million. The one analyst firm that has offered up projections has revenues falling to just under $305 million in FY2023 and profits dropping to $1.07 a share. They see flat earnings growth in FY2024 even as sales rise five percent.
To say Innoviva is a complex set of moving parts seems an understatement. It has new C-Suite leaders, sold a major part of its revenue stream last summer, has various subsidiaries, and multiple licensing agreements. The stock also has a fairly large short position, is mostly disliked in the analyst community, and does not pay a dividend.
Given down Innoviva, Inc. earnings growth this year and flat next, it is hard to justify getting involved in this quite complex story for 13 times forward earnings. Therefore, I am passing on making any investment recommendation around Innoviva.
If you want something new, you have to stop doing something old“― Peter Drucker.
Editor’s Note: This article discusses one or more securities that do not trade on a major U.S. exchange. Please be aware of the risks associated with these stocks.
Read the full article here





