Wave Life Sciences Ltd. (NASDAQ:WVE) develops stereopure oligonucleotides using its PRISM platform to target disease-causing genetic mutations. This is its pipeline:
WVE PIPELINE (WVE WEBSITE)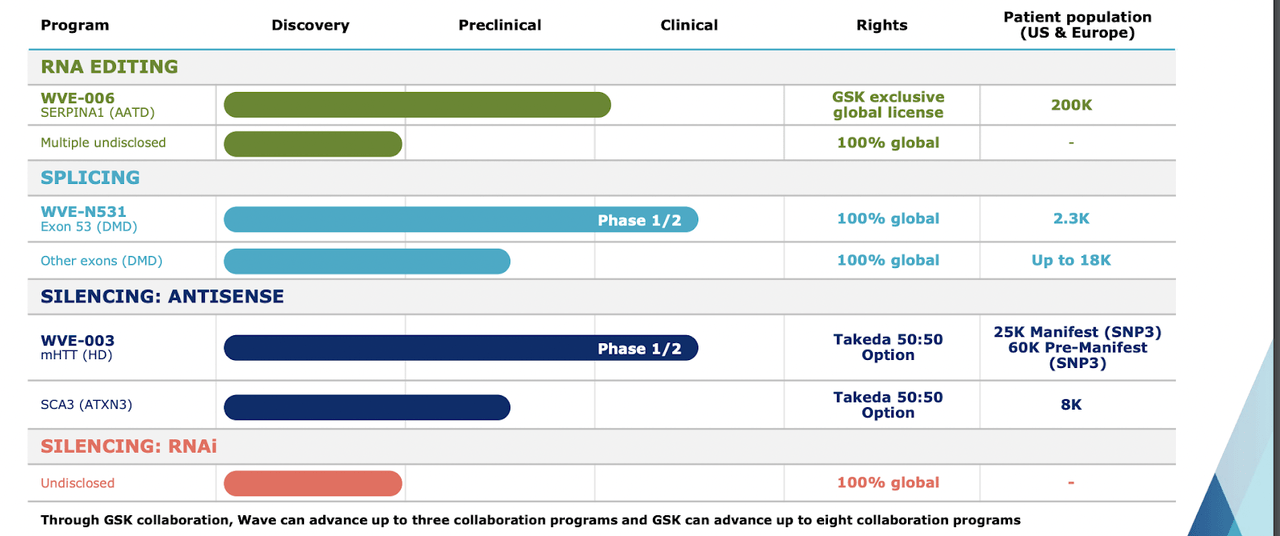
Lead assets are WVE-N531 targeting exon 53 skipping DMD, and WVE-003 targeting Huntington’s Disease in phase 1/2 trials. WVE owns the first molecule globally, while Takeda has a 50/50 option for the second one. There’s a third molecule in the clinic, owned by GSK globally, which is WVE-006 targeting Alpha-1 antitrypsin deficiency (AATD). Other assets are in preclinical stages.
The company’s technology provides for three things – RNA editing, splicing and silencing.
WVE tech (WVE website)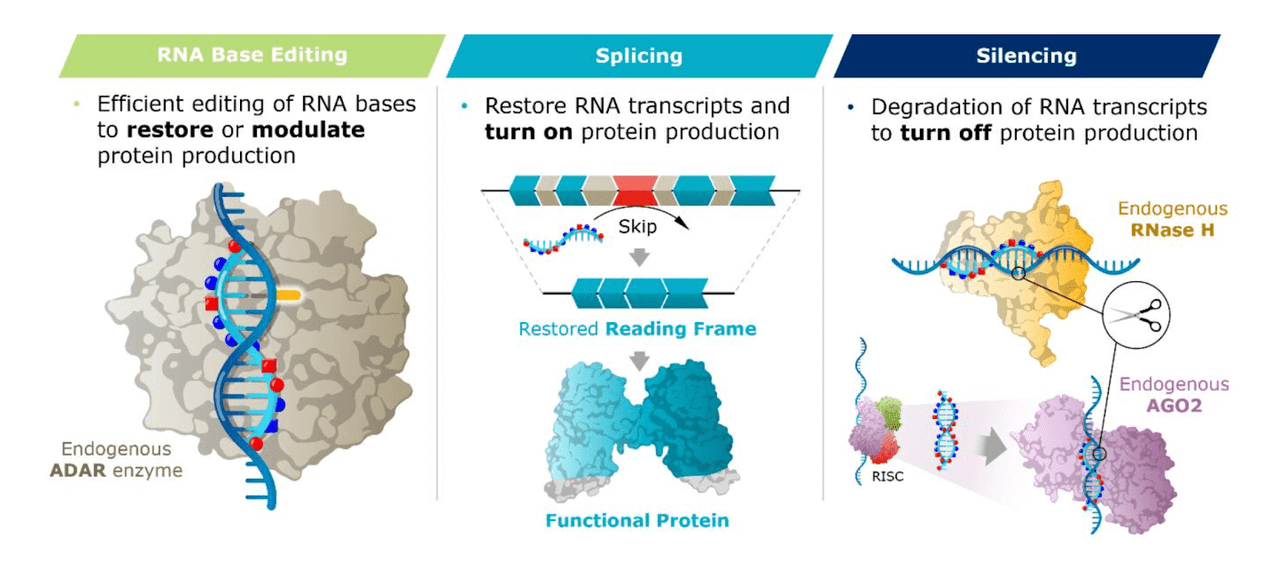
Thus, each of the three molecules in the clinic represents one of these three technologies. WVE-N531 uses RNA splicing, WVE-006 uses base editing, and WVE-003 works through gene silencing.
The base editing they use is from adenosine to inosine, or A-to-I, the most common form of RNA editing. The technology uses oligonucleotides (“AIMers”) for the editing, and the company claims that this gives them “access to areas of disease biology that are not viable for other therapeutic modalities.”
The company has two major collaborations, with GSK plc (GSK) and Takeda (TAK). The GSK deal was stuck in December 2022 and here are the details of payments:
Under the terms of the GSK Collaboration Agreement, we received an upfront payment of $170.0 million, which included a cash payment of $120.0 million and a $50.0 million equity investment. In addition, assuming WVE-006 and GSK’s eight collaboration programs achieve initiation, development, launch, and commercialization milestones, we would be eligible to receive up to $3.3 billion in cash milestone payments…
The Takeda program is older, from 2018, and the terms are as follows:
In April 2018, the Takeda Collaboration became effective and Takeda paid Wave $110.0 million as an upfront payment. Takeda also agreed to fund our research and preclinical activities in the amount of $60.0 million during the four-year research term and to reimburse Wave for any collaboration-budgeted research and preclinical expenses incurred by us that exceed that amount.
The Takeda deal was foundational, but the GSK deal is potentially huge. Under this deal, GSK has an exclusive global license to WVE-006 for AATD. GSK can advance up to eight collaboration programs, while WVE up to 3 collaboration programs using GSK’s genetic “insight.” The lead indication under this deal is WVE-006, targeting AATD, which is “designed to correct mutant SERPINA1 transcript to address both liver and lung manifestations of AATD.” In preclinical testing, WVE-006 was seen to decrease lobular inflammation in the liver, and also reduces PAS-D globular size, where Periodic Acid-Schiff (PAS’) with diastase (PAS-D) is a stain used to detect AATD.
WVE’s proprietary lead molecule is WVE-N531, which is targeting the rare exon 53 skipping DMD, which makes up some 8-10% of the already rare DMD population. Whichever exon you skip, the ultimate goal is to produce functional dystrophin, and the FDA considers this as an adequate surrogate endpoint for accelerated approval. In preclinical trials with nonhuman primates, WVE-N531 was found to attain high concentration in the heart and diaphragm, and lead to 71% restoration of dystrophin.
Last year, in December, WVE provided positive proof of concept data from the initial cohort of the Phase 1b/2a proof-of-concept study of WVE-N531 in three boys with Duchenne muscular dystrophy (“DMD”) amenable to exon 53 skipping. From our TickerBay software, here’s the data:
The efficacy data from the Phase 1b/2a proof-of-concept study of WVE-N531 in three boys with Duchenne muscular dystrophy (DMD) amenable to exon 53 skipping are as follows: – Mean exon skipping of 53% (range: 48-62%) was observed after three consecutive doses of WVE-N531. – Mean dystrophin production was measured to be 0.27% of normal as measured by Western blot. However, it was below the level of quantification (BLQ: 1%), indicating that dystrophin protein production was very low. – Muscle concentrations of WVE-N531 were high, with a mean tissue concentration of 42 micrograms/gram (6.1 micromolar). – RNAscope results indicated that WVE-N531 is reaching the nucleus in muscle cells. It’s important to note that these results are from an initial cohort and represent early evidence of WVE-N531’s efficacy. Further research and longer exposure will be needed to confirm the promise of these early data.
The company is now initiating part B, a potentially registrational phase 2 trial. Data is expected in 2024.
For the third candidate, base editing molecule WVE-003 targeting Huntington’s Disease, preclinical proof of concept data was published in Nature in March last year. Here’s the abstract:
Technologies that recruit and direct the activity of endogenous RNA-editing enzymes to specific cellular RNAs have therapeutic potential, but translating them from cell culture into animal models has been challenging. Here we describe short, chemically modified oligonucleotides called AIMers that direct efficient and specific A-to-I editing of endogenous transcripts by endogenous adenosine deaminases acting on RNA (ADAR) enzymes, including the ubiquitously and constitutively expressed ADAR1 p110 isoform. We show that fully chemically modified AIMers with chimeric backbones containing stereopure phosphorothioate and nitrogen-containing linkages based on phosphoryl guanidine enhanced potency and editing efficiency 100-fold compared with those with uniformly phosphorothioate-modified backbones in vitro. In vivo, AIMers targeted to hepatocytes with N-acetylgalactosamine achieve up to 50% editing with no bystander editing of the endogenous ACTB transcript in non-human primate liver, with editing persisting for at least one month. These results support further investigation of the therapeutic potential of stereopure AIMers.
Data showed that AIMers were present in NHP liver by day 50, and there was substantial and durable, and highly specific, editing in vivo. The company plans to produce more clinical data this year and take the discussion with Takeda to their next steps.
Financials
WVE has a market cap of $465mn and a cash balance of $170mn. Research and development expenses were $33.3 million in the second quarter of 2023, while general and administrative expenses were $12.3 million. That gives them a runway of 4-5 quarters.
WVE stock is substantially held by institutions and other smart money players, with retail holding being at 5%. Key holders are RA Capital, GSK PLC and Shin Nippon. Insider transactions are almost all sell transactions.
Risks
WVE’s cash balance is low, and given the early stage of development, they will need more funds. While GSK is funding some of their R&D, they will still need to raise funds very soon – and it may not all be non-dilutive.
The other risk is the early stage of the pipeline. While there is clinical DMD data, and arguably, DMD trials are usually small because of the rareness of the disease, yet, all they have is data from 3 patients.
Earlier this year, the stock crashed after an asset called WVE-004 failed a Phase 1/2 trial for certain patients with amyotrophic lateral sclerosis (ALS) and dementia, where it was unable to beat placebo.
Bottom Line
I found WVE interesting. These gene editing companies have a tough time ahead of them managing unwanted side effects and so on. But this is the future of medicine, and WVE seems to be creating some of it. I will stay interested.
Editor’s Note: This article discusses one or more securities that do not trade on a major U.S. exchange. Please be aware of the risks associated with these stocks.
Read the full article here






