Genelux (NASDAQ:GNLX) is an immuno-oncology frontrunner that presents a promising investment opportunity in the fight against cancer. With its cutting-edge technology platform, strong clinical pipeline, and robust financial position, Genelux is poised for long-term growth and success in the rapidly evolving oncolytic viral immunotherapy landscape.
As a late-stage clinical biopharmaceutical company, Genelux focuses on using advanced oncolytic viral immunotherapies to combat solid tumors. Its primary candidate, Olvi-Vec, embodies a revolutionized approach to cancer treatment, leveraging specifically engineered oncolytic vaccinia viruses capable of targeting and destroying cancer cells while leaving healthy tissues unscathed.
After securing over $65 million in financing throughout 2023 and conducting successful clinical trials backed by strong investor support, Genelux has displayed an unwavering commitment to advancing its clinical programs. This includes Olvi-Vec’s pivotal Phase 3 trial for platinum-resistant/refractory ovarian cancer (PRROC) and an upcoming Phase 2 trial for recurrent non-small cell lung cancer. Moreover, with promising top-line outcomes published in JAMA Oncology, these results reaffirm the company’s ability to deliver promising therapeutic options and add significant value to the immuno-oncology market.
Recent Placement Developments
The recent private placement of 900,000 shares valued at $20.00 each, amassing a substantial $18 million in gross proceeds, demonstrates the strong investor belief in Genelux’s bright future. Moreover, the lack of a placement agent highlights the authentic and inherent interest in the company’s mission.
Following the previously disclosed $33 million private placement on May 15, 2023, the total capital raised by Genelux in 2023 surpasses an impressive $65 million. This extraordinary achievement not only underscores the steadfast faith of existing investors but also highlights the company’s remarkable financing abilities, which are crucial for its long-term triumph.
Upon the expected final closing of the private placements, Genelux’s cash runway is projected to extend into the first quarter of 2026. This provides the company with a solid financial position to actively pursue its objectives and strategies, propelling the company to new levels of accomplishment.
The primary objective of Genelux’s use of proceeds from the private placements is to expand manufacturing capabilities, accelerate clinical programs, including the pivotal Phase 3 registrational trial of Olvi-Vec for platinum-resistant/refractory ovarian cancer patients, and ensure adequate working capital and general corporate purposes. These allocations undoubtedly manifest the company’s unwavering dedication to expansion and strengthening its foothold in the competitive immuno-oncology arena.
The initial closing of the private placement, anticipated to be approximately $5.5 million, is scheduled to take place on or before June 20, 2023. The remaining committed funds, totaling roughly $12.5 million, will be received on or before November 15, 2023, at the same price as the initial closing investments. This phased financing approach offers the company a consistent flow of capital, guaranteeing that Genelux can effectively meet its clinical and operational demands.
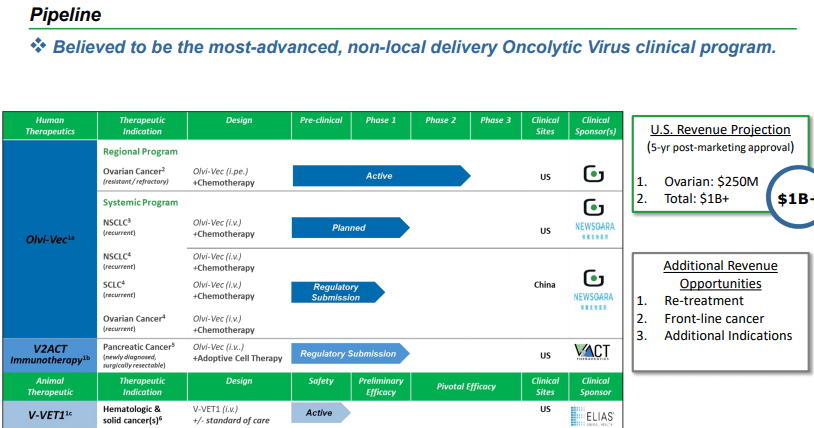
Product Pipeline
Genelux’s primary candidate, Olvi-Vec infiltrates and annihilates cancer cells without harming healthy cells. This virus also aids the immune system in identifying and removing cancerous cells and can be equipped with supplementary genes to bolster its therapeutic influence.
Olvi-Vec has been studied in clinical trials featuring nearly 150 patients with various forms of cancer, using both regional and systemic methods and in conjunction with additional treatments. These trials have emphasized the excellent tolerability of the virus, its capacity to penetrate and multiply within distant tumor sites, and its effectiveness against circulating tumor cells. Furthermore, the virus has been found to evoke a pro-inflammatory response, activate adaptive immunity, and display remarkable anti-tumor activity, consequently leading to enhanced tumor shrinkage, progress-free survival, improved overall survival rates, and better quality of life for patients.
investors.genelux.com
Currently, Genelux is progressing with a critical Phase 3 trial, dubbed OnPrime, which examines the potency and safety of Olvi-Vec combined with platinum-doublet and bevacizumab for patients afflicted with platinum-resistant/refractory ovarian cancer. This study involves 450 participants across numerous worldwide locations. In addition, the company is organizing a Phase 2 trial to explore Olvi-Vec’s effects on individuals suffering from recurrent non-small cell lung cancer.
Beyond Olvi-Vec, Genelux is cultivating a multitude of preclinical product candidates using their innovative CHOICE discovery platform. These candidates consist of modified vaccinia viruses that express genes such as hNIS, CEA, IL-12, GM-CSF, IL-15, TRAIL, IL-24, IL-2, and IL-21. The specified genes play a critical part in augmenting the anti-tumor activity or immune response toward cancer cells.
Furthermore, the company has crafted a silk-elastin-like protein polymer matrix intended for the intraoperative administration of oncolytic vaccinia virus, which enhances the retention and distribution of the virus within tumor sites and potentially optimize therapeutic results. Genelux’s preclinical pipeline has displayed encouraging outcomes in animal models of varying cancer forms, and their research has been published in a variety of peer-reviewed articles. These achievements underline the company’s dedication to propelling the domain of oncolytic viral immunotherapy.
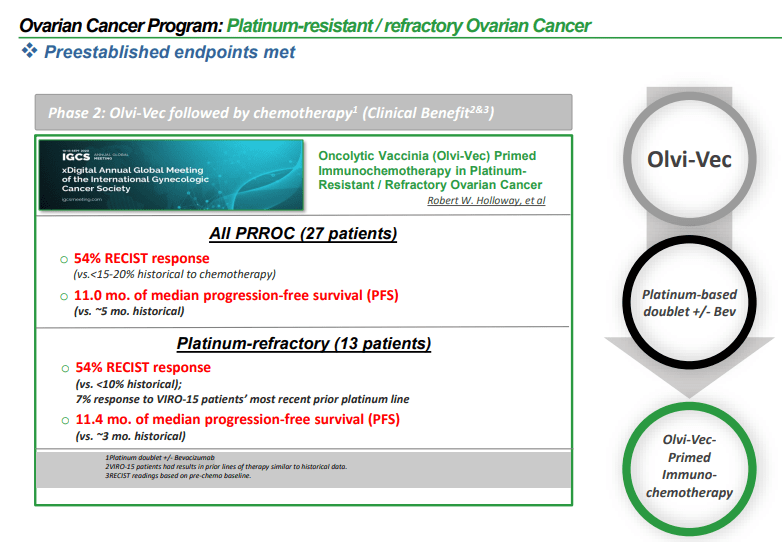
Promising Results: Olvi-Vec Therapy for Ovarian Cancer
Genelux announced encouraging top-line outcomes from its Phase 2 VIRO-15 study in JAMA Oncology. The trial focused on Olvi-Vec-enhanced immunochemotherapy for patients with platinum-resistant/refractory ovarian cancer who had previously undergone extensive treatments. The published results support the effectiveness and safety of Olvi-Vec while strengthening the company’s role in ovarian cancer therapy.
The Phase 2 clinical trial results indicate success for platinum-resistant/refractory ovarian cancer patients with limited treatment choices and urgent medical needs. In a group of 27 patients who had experienced an average of four prior lines of therapy, the treatment regimen of Olvi-Vec followed by platinum-based chemotherapy, with or without bevacizumab, resulted in an objective response rate (ORR) of 54% and median progression-free survival (PFS) of 11 months, all while maintaining a manageable safety profile.
investors.genelux.com
Key findings showed that all patients completed both Olvi-Vec infusions and chemotherapy, with an average follow-up period of 47 months. The ORR according to RECIST1.1 criteria was 54%, and the disease control rate reached 88%. Moreover, 86% of evaluable patients experienced tumor reduction, and 96% presented lower CA-125 levels. Median PFS by RECIST1.1 standards was 11 months for all patients, consisting of 10 months for the platinum-resistant subgroup and 11.4 months for the platinum-refractory subgroup. Furthermore, overall survival rates (OS) across both groups were 15.7 months, with the platinum-resistant subgroup recording a median OS of 18.5 months and the platinum-refractory subgroup at 14.7 months.
It is worth noting that the most prevalent treatment-related adverse events were fever and abdominal discomfort; however, there were no grade 4 treatment-related adverse events, treatment-related discontinuations, or treatment-related deaths.
These advancements bode well for the company, as they emphasize the significant progress achieved with Olvi-Vec and solidify its potential as an effective treatment for platinum-resistant/refractory ovarian cancer patients. The positive outcomes also reflect Genelux’s commitment to innovation and pursuit of dominating the immuno-oncology market.
Addressing Risks and Concerns
Olvi-Vec employs a modified oncolytic vaccinia virus that selectively infects and kills tumor cells without damaging normal cells. However, the innovative nature of this approach raises several valid concerns. First and foremost, accurately targeting tumor cells is essential to avoid causing unintended harm to healthy tissues. Although Olvi-Vec has displayed selectivity in clinical trials, there still remains a potential risk of off-target effects or unplanned viral replication in normal cells, which could result in adverse effects and constraints on the overall safety of the treatment.
Another concern relates to the potential development of viral resistance. Viruses, including oncolytic viruses, possess the capability to evolve and establish resistance mechanisms. The replication of Olvi-Vec within tumor cells might provide a chance for resistant tumor cell clones to emerge and become impervious to the virus’s therapeutic effects. Closely monitoring and addressing viral resistance is crucial for securing the long-term effectiveness of Olvi-Vec.
Additionally, challenges may arise concerning the immune system’s response to the viral therapy. Olvi-Vec is engineered to stimulate the immune system to detect and remove tumor cells. However, the immune system response can be intricate and constantly changing, with the potential for immunosuppression or the development of immune escape mechanisms by cancer cells. Comprehending and managing the complex interaction between the virus, the immune system, and the tumor microenvironment is vital for optimizing the therapeutic efficacy of Olvi-Vec.
Moreover, it is important to consider the possibility of adverse immune reactions or inflammatory responses to viral therapy. While Olvi-Vec has demonstrated proinflammatory activity and activation of adaptive immunity, it is crucial to carefully manage the immune response. Excessive or uncontrolled immune response might lead to unintended consequences, such as severe inflammation, cytokine release syndrome, or autoimmune reactions. Striking a balance in the immune response to achieve optimal therapeutic outcomes while minimizing adverse effects is of utmost importance.
As for Genelux’s other product candidates in preclinical development, similar concerns regarding selectivity, off-target effects, viral resistance, and immune responses are relevant. Each candidate utilizes a modified vaccinia virus expressing different genes or cytokines to enhance anti-tumor activity or immune responses. The safety and efficacy of these candidates rely on addressing the same challenges faced with Olvi-Vec while considering the specific implications and potential risks associated with the expressed genes or cytokines.
Competitors
Genelux faces competition from other companies engaged in the development of oncolytic viral immunotherapies for cancer, such as Amgen, Oncolytics Biotech, TILT Biotherapeutics, and Replimune.
Amgen’s (AMGN) product Imlygic (talimogene laherparepvec) is an FDA-approved oncolytic herpes simplex virus-1 targeting melanoma, receiving approval in 2015. While Imlygic has demonstrated success in melanoma treatment, it is limited to local injections for accessible tumor lesions, and its efficacy and safety for other solid tumor types remain uncertain. Genelux’s Olvi-Vec, on the other hand, encompasses broader application prospects, targeting numerous solid tumor types and enabling both regional and systemic administration.
Reolysin (pelareorep) a proprietary variant of the reovirus developed by Oncolytics Biotech (ONCY), has demonstrated clinical activity and safety in various cancer types, with a particular emphasis on multiple myeloma and breast cancer. In contrast, Genelux’s Olvi-Vec has exhibited promising results across different cancer forms, including platinum-resistant/refractory ovarian cancer and recurrent non-small cell lung cancer, highlighting its broader applicability.
TILT Biotherapeutics is developing oncolytic adenoviruses armed with cytokines and checkpoint inhibitors. Despite a strong preclinical pipeline, the company has yet to progress to later-phase clinical trials, whereas Genelux’s Olvi-Vec is in a pivotal Phase 3 trial, reflecting advanced stages of development and a closer path to potential approval and market entry.
Replimune’s (REPL) oncolytic viruses are derivatives of the herpes simplex virus, comprising the lead candidate RP1 in Phase 2 trials for melanoma, non-melanoma skin cancer, and MSI-H/dMMR solid tumors. While the platform has potential, its application remains narrow compared to Genelux’s extensive preclinical pipeline generated by the CHOICE platform, featuring various cytokine-enhanced vaccinia viruses.
Conclusion
Genelux’s outstanding achievements in immune oncology indicate a bright financial outlook for the company. With strong investor support and a proven ability to secure substantial funding, Genelux is in a robust financial position to aggressively pursue its ambitious objectives and innovative strategies. These accomplishments, along with a solid balance sheet and extended cash runway, position the company for continued growth.
The company’s dedication to advancing its clinical programs, particularly the pivotal Phase 3 trial of Olvi-Vec, demonstrates a commitment to securing a strong foothold in the oncology market. The recent top-line outcomes published in JAMA Oncology only serve to emphasize Genelux’s potential to carve a niche in the competitive immuno-oncology arena.
Read the full article here