An exemplar of innovation and successful product development in the pharmaceutical sector, Immunocore Holdings (NASDAQ:IMCR) is at the precipice of revolutionizing the biotechnology industry with its unique T cell receptor-based bispecific immunotherapies. Given the robust sales and promising clinical trial results, Immunocore has proven that it is on a lucrative growth trajectory. We believe that the company’s unwavering commitment to research and development (R&D), ambitious investments for market expansion, and diversified pipeline targeting several unmet medical needs indicate that Immunocore is poised for exceptional long-term growth. Consequently, we are convinced that an investment in Immunocore Holdings is an opportunity to benefit from the relentless pursuit of progress in the biopharmaceutical space.
The biopharmaceutical sector is a fiercely competitive battleground, where only a few companies can establish a lasting impact. Immunocore Holdings is an ascending biotech firm that shows all the hallmarks of becoming a market leader with its cutting-edge T cell receptor (TCR) bispecific immunotherapies that aim to conquer cancer, autoimmune diseases, and infectious illnesses. Positioned at the forefront of disruptive immunotherapy, the company has an impressive line-up of drugs, spearheaded by the trailblazing KIMMTRAK, which has been approved in multiple countries and boasts skyrocketing sales figures. Riding on the wings of ingenuity and enterprise, Immunocore’s ambitious expansion plans, a treasure trove of cash reserves, and striking investments in research and development signal the company’s confidence and foresight to realize long-term success. As Immunocore continues to break new ground in targeted therapy, now is our chance to seize this rare investment opportunity and capitalize on the company’s potential to become an indomitable force in the continually evolving world of biotechnology.
Financials Continue to Grow
Immunocore Holdings has displayed remarkable financial outcomes that demonstrate its exceptional market position. Largely fueled by the sales of KIMMTRAK, an innovative treatment alternative gaining prominence in the field, the company’s financial future appears quite bright.
In the first quarter of 2023, Immunocore posted an impressive £42.1 million ($52.0 million) in KIMMTRAK revenue, signifying a nearly astronomical increase in sales in comparison to the £10.5 million documented in the equivalent timeframe in 2022. Moreover, the company’s steady performance demonstrated by equivalent revenue levels in Q4 of 2022 and Q1 of 2023 reveals that Immunocore is not only expanding, but is doing so in a sustainable manner.
Furthermore, the company’s devotion to research and development is praiseworthy, as expenses reached £28.4 million (or $35.2 million) during Q1 2023, up from £18.6 million in the previous year. This notable growth in investment underscores the company’s unwavering commitment to advancing and enhancing its treatments to keep its products at the vanguard of the industry.
Although it’s undeniable that the growth in selling and administrative costs to £33.3 million (or $41.2 million) in 2023 from £20.1 million in 2022 is substantial, it showcases the company’s continuing efforts to broaden its international reach and augment its market share. The escalation in expenditures signifies strategic and ambitious long-term planning, reflecting the company’s determination to guarantee ongoing success.
Despite witnessing a total operating loss of £17.4 million (or $21.5 million) during Q1 2023, it’s crucial to comprehend the broader context. As of March 31, 2023, Immunocore’s substantial cash reserve of £337.5 million (or $417.4 million), in contrast to £332.5 million as of December 31, 2022, presents an invaluable safety net for any potential financial obstacles the company might encounter.
Valuable T-Cell Receptor Products
Immunocore specializes in creating unique T cell receptor bispecific immunotherapies aimed at treating a variety of illnesses, including cancer, autoimmune diseases, and infectious diseases. The company uses ImmTAC product candidates to harness the power of the immune system in eradicating abnormal cells, leading to exceptional results. By 2023, Immunocore’s product lineup includes its leading product KIMMTRAK, the first TCR bispecific therapy to display an overall survival advantage in a randomized Phase 3 trial. Approved to treat metastatic uveal melanoma, KIMMTRAK targets the gp100 protein, directing T cells to eliminate uveal melanoma cells.
Immunocore IR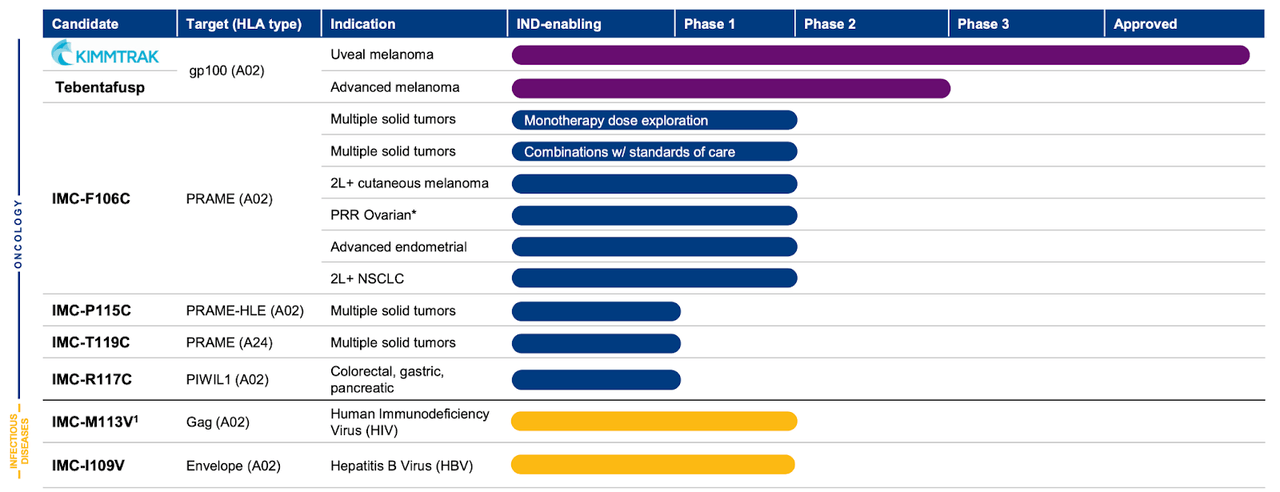
Additionally, Immunocore is studying the potential of KIMMTRAK to address advanced cutaneous melanoma. Furthermore, the company’s pipeline features IMC-F106C, an innovative ImmTAC targeting PRAME, a cancer-testis antigen discovered in several solid tumors and hematological malignancies. Presently in Phase 1/2 clinical trial, IMC-F106C is being assessed both as a standalone treatment and in conjunction with pembrolizumab for PRAME-positive solid tumors therapy. The trial data is expected to be available by the first half of 2024.
Other promising product candidates encompass PRAME-A24 ImmTAC, targeting PRAME HLA-A24 and projected to undergo investigational new drug (IND)-enabling research in 2023, and PRAME-A02 HLE ImmTAC, which focuses on PRAME HLA-A02 using a half-life extended (HLE) format and is anticipated to enter IND-enabling studies in 2023. Lastly, the groundbreaking ImmTAC, PIWIL1 ImmTAC, aims at PIWIL1, an oncogenic protein associated with colorectal and other gastrointestinal cancers. Immunocore intends to initiate IND-enabling research for PIWIL1 ImmTAC in Q4 2023, showcasing a wide-ranging and impressive product portfolio.
Impressive KIMMTRAK Result
Immunocore Holdings exhibited four posters featuring KIMMTRAK data for metastatic uveal melanoma (mUM) patients with HLA-A*02:01.
Approved in over 30 nations, KIMMTRAK has aided numerous patients. The data from the Phase 3 trial displayed at the conference emphasizes the solid connection between circulating tumor DNA (ctDNA) reduction and enhanced survival following KIMMTRAK therapy. With almost four years of follow-up from the Phase 2 trial, this signifies the most extensive and longest-lasting examination of survival and safety for any TCR treatment.
Immunocore IR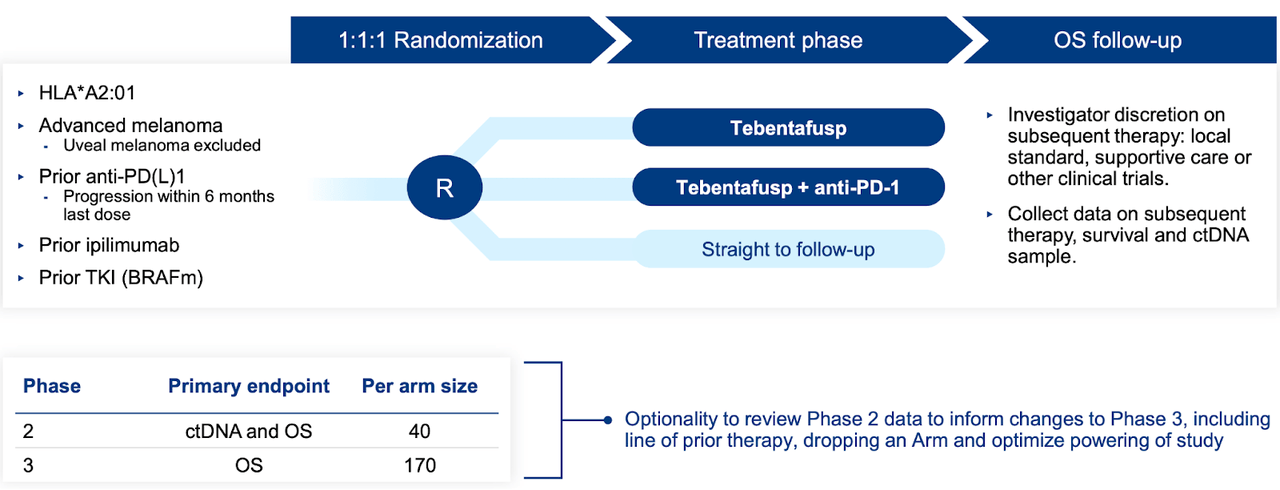
In the Phase 3 and Phase 2 trials, ctDNA reduction was seen in 88% by week 9 and 71% of mUM patients, respectively, and correlated with a more extended overall survival (OS). A higher percentage of ctDNA clearance was also detected in first-line patients compared to previously treated patients. The Phase 3 trial ctDNA clearance correlated with an 84% OS rate after one year. The connection between ctDNA reduction and more extended OS proved to be a better long-term survival predictor than radiographic response, even in individuals with the best RECIST progressive disease (PD) response.
Immunocore IR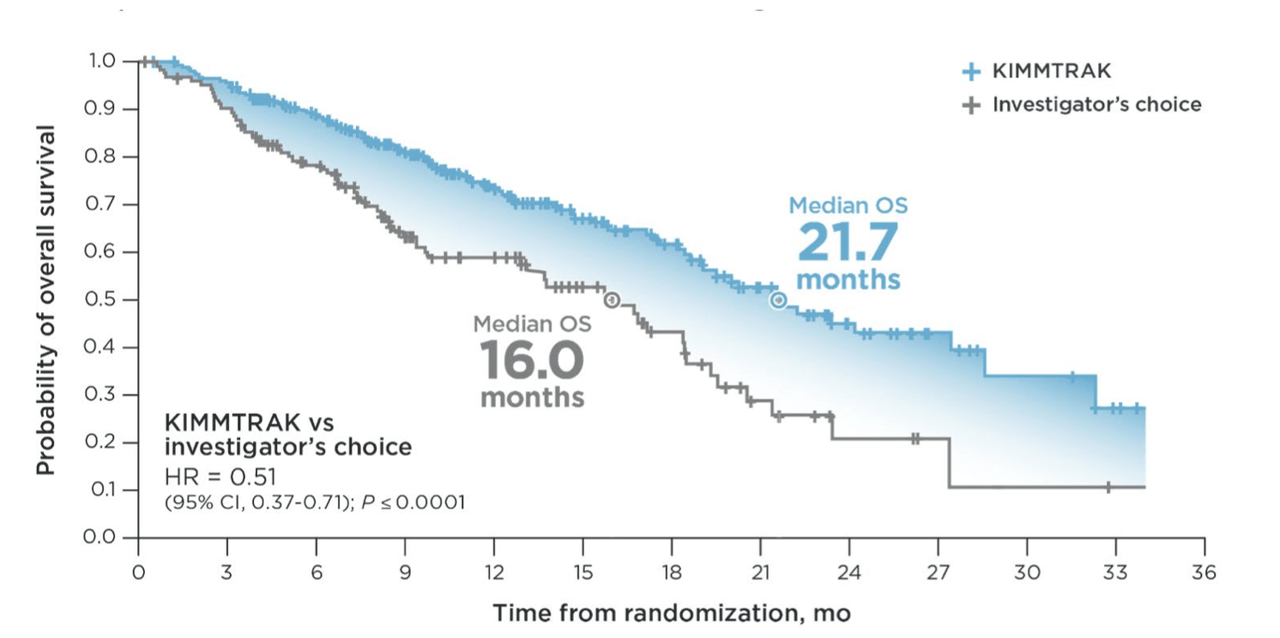
The ultimate analysis from the Phase 2 trial, following 46 months of follow-up, revealed a median OS of 16.8 months, in line with earlier data updates. The landmark OS rates were approximately double the historical rates in this specific patient population. During the primary analysis in 2020, the median OS for KIMMTRAK-treated patients was 21.7 months, compared to 16.0 months for those treated with the investigator’s choice.
Moreover, a collective analysis from three clinical trials found KIMMTRAK showed activity in intraocular lesions in 12 mUM patients with orbital disease, endorsing its use for individuals with unresectable uveal melanoma. Additionally, in vitro data offered insights into how KIMMTRAK might contribute to OS advantages, even in tumors with inconsistent gp100 expression.
These outcomes emphasize the importance of KIMMTRAK and its potential to significantly influence metastatic uveal melanoma treatment. The data presented at the conference highlights its considerable advantages and the bright future for this TCR bispecific immunotherapy. As the biotechnology company continues to research and develop groundbreaking treatments for cancer and other illnesses, Immunocore’s revolutionary work is expected to make a substantial difference in patients’ lives globally.
Mechanism-Related Risks
KIMMTRAK redirects T cells to eliminate uveal melanoma cells, and demonstrates promising results in clinical trials. However, one potential concern is the specificity of the targeting mechanism. If the drug inadvertently recognizes healthy cells expressing gp100, it could lead to unintended immune responses and harm to normal tissues. Careful evaluation of potential off-target effects and drug selectivity will be crucial for success.
Immunocore IR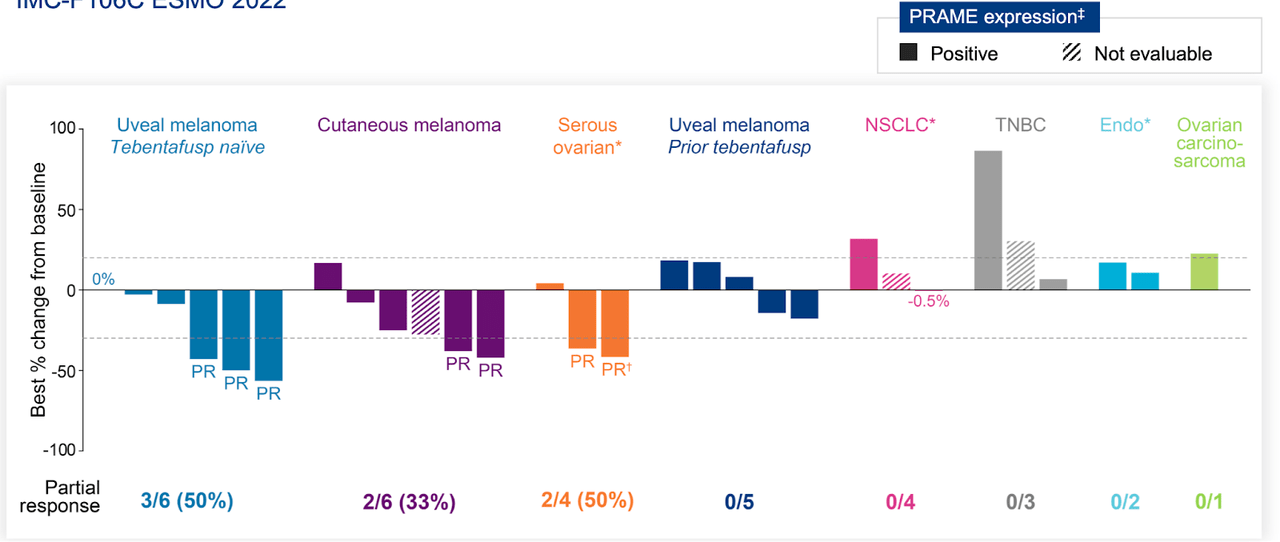
IMC-F106C’s success relies on specific recognition of PRAME HLA-A02, which is present in about 50% of the population with hematological malignancies. However, the potential concern here is the heterogeneous expression of PRAME within tumors. If a tumor has a mixed population of cells with varying levels of PRAME expression, the drug may not effectively eliminate all cancer cells, leading to an incomplete response or disease recurrence. Accurately assessing PRAME expression levels within individual tumors to maximize therapeutic efficacy is vital.
PRAME-A24 ImmTAC aims to broaden the patient population by targeting PRAME HLA-A24, another variant of PRAME. While expanding the treatment scope is commendable, the lower prevalence of PRAME HLA-A24 in the population (approximately 20%) raises a potential concern. This reduced frequency may limit the number of eligible patients who can benefit from this particular drug. Adequate patient selection and screening processes are crucial for PRAME-A24 ImmTAC’s success, and its ability to reach a meaningful patient population.
PRAME-A02 HLE ImmTAC employs a half-life extended format to improve the pharmacokinetic profile and patient convenience. This approach focuses on reducing dosing frequency and infusion time at the potential risk of compromising the drug’s efficacy. Altering the drug’s properties to extend its half-life might impact its ability to effectively engage the immune system and eliminate cancer cells. Striking a balance between extended half-life and optimal therapeutic activity is essential for PRAME-A02 HLE ImmTAC’s success.
Regarding PIWIL1 ImmTAC, the complexity and heterogeneity of these tumor types raise a potential concern. Cancers often exhibit genetic and phenotypic diversity, leading to variations in PIWIL1 expression and activity. This heterogeneity could affect the drug’s ability to engage and eliminate cancer cells effectively in every patient. Further, understanding the interplay between PIWIL1 expression and drug response and the thorough characterization of tumor types and patient selection will be critical for PIWIL1 ImmTAC’s success.
Innovation Overtakes Competitors
Immunocore’s innovative TCR bispecific immunotherapies are in competition with various other immunotherapies, such as chimeric antigen receptor T-cell (CAR-T) therapy, T cell engager BITE molecules, and checkpoint inhibitors. Each competitor’s technology has its advantages and disadvantages, but Immunocore’s products demonstrate unique features that make them stand out in the field.
Immunocore IR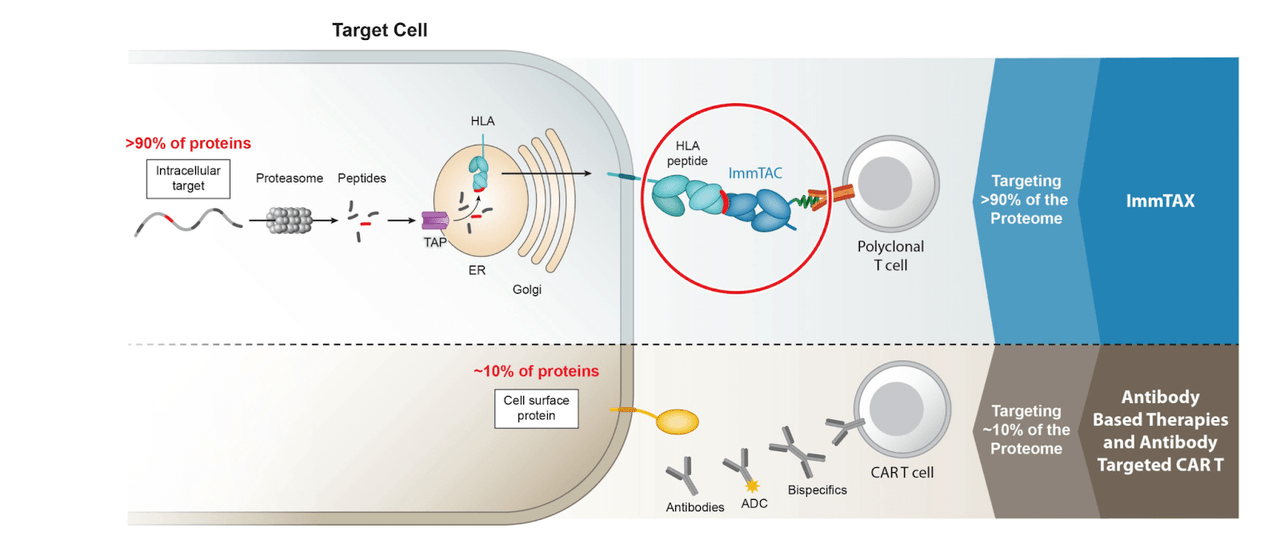
In comparison to CAR-T therapy, Immunocore’s ImmTACs offer several advantages. While CAR-T technology involves genetically engineering a patient’s T cells to recognize cancer cell surface antigens, ImmTACs are off-the-shelf bispecific molecules that bridge T cells to cancer cells via their natural TCRs, targeting intracellular antigens presented on the cell surface by Major histocompatibility complex MHC molecules. Because ImmTAC can access a wider range of targets than CAR-T, including intracellular antigens, they offer a broader scope for therapeutically relevant targets. Additionally, the off-the-shelf nature of ImmTACs provides a more accessible and cost-effective treatment option compared to the complex and personalized CAR-T therapies.
Regarding BiTE molecules, these small bispecific molecules also bridge T cells to cancer cells, similar to ImmTACs. However, BiTEs primarily target cell surface antigens, whereas Immunocore’s ImmTACs are designed to target both surface-expressed and intracellular antigens presented by MHC molecules. This ability to target intracellular antigens grants ImmTACs access to a wider array of targets for potential therapy, thus setting them apart from BiTE competitors.
When compared to checkpoint inhibitors, which focus on blocking immune checkpoint molecules like PD-1, PD-L1, or CTLA-4 to unleash the immune response against cancer cells, Immunocore’s ImmTAC actively redirects T cells to eliminate specific cancer cells expressing the target antigen. By assuming a more targeted and controlled approach, ImmTACs can potentially reduce the risk of off-target side effects and immune-related adverse events that have been observed with some checkpoint inhibitors. Moreover, the ability to target antigens expressed exclusively on cancer cells may augment the chance of a diverse immune response, potentially providing better treatment outcomes.
Conclusion
In conclusion, Immunocore Holdings stands as a paragon of innovation and success in the biopharmaceutical sector, orchestrating a captivating symphony of targeted therapies that hold the potential to create an indelible mark in the industry. We are confident that the company’s financial future shines brightly, reflecting its unwavering commitment to research and development, sustainability-driven sales growth, and strategic investments in market expansion. A diversified and robust pipeline, led by the revolutionizing KIMMTRAK, embodies the company’s ambitious vision to tap into the immune system’s untapped potential.
As Immunocore continues to push the boundaries of what is possible in targeted immunotherapies, we posit that now is a golden opportunity to invest in a company poised for prodigious long-term growth. It is our firm belief that an investment in Immunocore Holdings will bear fruit in the form of unparalleled success, as we stand shoulder-to-shoulder with a company that dares to challenge the status quo and rewrite the history of healthcare and medicine.
Read the full article here






