As a market leader in the genetic testing sector, Natera’s (NASDAQ:NTRA) innovative and diversified product portfolio sets the company apart from competitors and positions it for considerable long-term growth. The company’s strong financial standing, unique focus on precision medicine, and commitment to continued innovation make Natera a compelling investment opportunity for those seeking exposure to the rapidly evolving healthcare space.
The healthcare industry is experiencing a paradigm shift, with precision medicine and early detection taking center stage, and Natera is at the forefront of this transformation. The company’s cutting-edge suite of products, including Panorama, Prospera, and Signatera, are not only helping to revolutionize prenatal testing, transplant monitoring, and cancer surveillance, but are also outpacing the offerings of well-established competitors. Imbued with a steadfast dedication to innovation, excellence, and market expansion, Natera continues to demonstrate impressive revenue growth and positive financial prospects. As the firm forges ahead, bolstered by recent legal victories and a strong intellectual property arsenal, savvy investors would be hard-pressed to overlook the potential rewards that this groundbreaking genetic testing leader offers.
Impressive Financial Performance
Natera has unveiled its financial performance for the initial quarter of 2023, highlighting an exceptionally strong and optimistic future for the organization. With overall revenues soaring to $241.8 million from $194.1 million in the corresponding time-frame in 2022, the firm has displayed a phenomenal 24.5% increase, surpassing doubters and cementing its position as a formidable presence in the sector.
investor.natera.com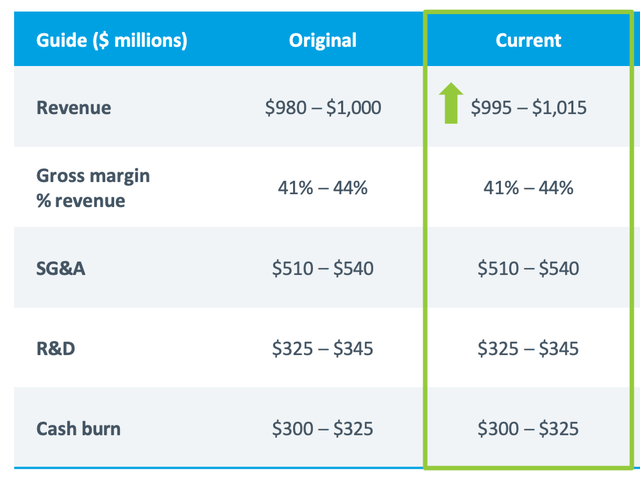
The remarkable expansion story continues, as Natera’s product revenues increased dramatically to $237.8 million from $190.0 million in 2022, marking a significant 25.2% rise. It’s evident that the enterprise is not complacent-its unwavering commitment to revolutionizing genetic testing has fueled the extraordinary escalation in test volumes, resulting in a 28% growth compared to the prior year.
Natera’s commitment to offering elite genetic testing services is evidenced by their capacity to handle an astounding 626,200 tests in just the first quarter of 2023. The test count growth trajectory remains strong, with an outstanding 27.9% upsurge from the initial quarter of 2022, fortifying their position as a key market contender.
Even though the first quarter of 2023 saw reduced margins mainly due to augmented labor, overhead expenses, and external services, Natera has effectively navigated these obstacles and emerged with a gross profit of $93.6 million. The organization’s total operational costs experienced a slight 1.7% growth, largely attributable to its tactical investments in personnel to support future expansion, volume enhancement, and product advancement.
As a result, the net loss for the first quarter of 2023 amounted to $136.9 million or ($1.23) per diluted share, which compares favorably with the net loss of $138.6 million or ($1.45) per diluted share in 2022. This further underlines Natera’s unwavering dedication to investing in the firm’s growth while optimizing shareholder value.
investor.natera.com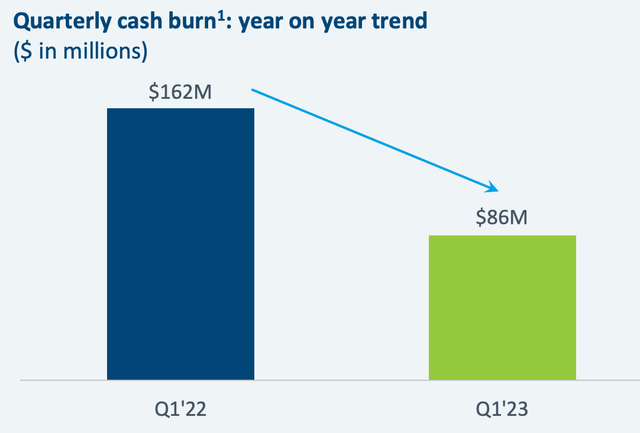
Natera’s robust financial standing as of March 31, 2023, is reflected in its possession of $812 million in cash, cash equivalents, short-term investments, and restricted cash. The company is raising its projections for 2023 total revenue to a new range of $995 million to $1.015 billion, a hopeful estimate that I believe signifies its self-assurance and aspiration to conquer the genetic testing market. Additionally, Natera maintains its predictions for a 2023 gross margin of approximately 41% to 44% of revenues.
A Plethora of Diverse Products
Natera offers a diverse range of innovative products, including Panorama, Prospera, and Signatera tests, each catering to specific medical needs. Panorama, a noninvasive prenatal testing (NIPT) product, uses single nucleotide polymorphism (SNP)-based technology to achieve high precision in detecting chromosomal abnormalities in both single and multiple pregnancies. Remarkably, this test can be conducted as early as the ninth week of pregnancy. Panorama’s distinguishing feature is its ability to differentiate between the expectant parents’ and the fetus’ DNA, enabling accurate screening for triploidy and avoiding errors affecting other NIPTs. However, it is essential to note that Panorama functions as a screening test and does not offer a conclusive diagnosis.
On the other hand, Prospera is a minimally invasive donor-derived cell-free DNA (dd-cfDNA) blood test specifically designed for monitoring heart transplant patients. This state-of-the-art product relies on over 13,000 pan-ethnic SNPs and advanced bioinformatics to deliver early warnings of active rejection risk, which can greatly influence patient care. Managing heart transplant recipients is a complex and challenging task, considering 25% of patients face acute rejection in their first year. Prospera helps mitigate these difficulties by providing a noninvasive method that reduces biopsy reliance, identifies both antibody-mediated rejection (AMR) and acute cellular rejection (ACR), and simplifies the procedure with a single blood draw.
Finally, Signatera is an innovative molecular residual disease assay (MRD). Its purpose is to offer personalized, highly sensitive detection of circulating tumor DNA (ctDNA) for individual patients, significantly improving early cancer relapse identification compared to standard care tools. The primary goal of Signatera is to monitor and track minimal residual disease in cancer-treated patients. MRD represents the minute number of cancer cells that may persist in the body after treatment, even during remission. Conventional techniques struggle to identify these remaining cancer cells, but their presence may indicate potential recurrence. By analyzing ctDNA-small fragments of tumor DNA found in the bloodstream-Signatera delivers an exceptionally sensitive method for detecting and monitoring residual cancer cells. Each patient receives a personalized Signatera test, utilizing custom-designed assays targeting mutations unique to their tumor, allowing for a remarkable level of sensitivity in detecting very low ctDNA levels in the blood.
Natera Wins Legal Battle
Natera recently achieved success in a patent violation lawsuit brought against ArcherDX/Invitae Corp (NVTA). A unanimous jury in the U.S. District Court for the District of Delaware confirmed that all products in question by ArcherDX/Invitae transgressed three of Natera’s patents. Consequently, the jury granted Natera a combined sum of $19.35 million, considering both past lost earnings and a 10% previous royalty fee. A following hearing will ascertain if an injunction against ArcherDX/Invitae will be enforced or if Natera will be awarded ongoing royalties.
This favorable legal outcome demonstrates Natera’s commitment to groundbreaking technology that enhances patient care, supported by empirical research. The violated patents constitute only a small section of Natera’s comprehensive global intellectual property collection, comprising more than 360 issued or pending patents, with over 180 related to oncology and MRD. Natera consistently dedicates itself to assisting the oncology society, serving both its doctors and patients. This judicial victory accentuates the firm’s status as an inventive power within the sector and bolsters its capacity to deliver state-of-the-art products and services.
Reliability and Accuracy Risks
Panorama screens for common chromosomal conditions in both singleton and twin pregnancies. Conducted as early as nine weeks into gestation, Panorama can differentiate between the DNA of the pregnant individual and the baby. This distinction allows the test to monitor triploidy and precludes sources of error in other NIPTs. However, Panorama serves as a screening test, not a definitive diagnostic tool, and may produce false positives or false negatives that can cause unnecessary anxiety or missed diagnoses.
On the other hand, Prospera is a noninvasive dd-cfDNA blood test developed for heart transplant monitoring, utilizing a multitude of pan-ethnic SNPs and advanced bioinformatics to provide early insights into active rejection risks in heart transplant recipients. Prospera reduces invasive biopsy needs and detects both AMR and ACR, offering a noninvasive alternative for patient monitoring. Nevertheless, concerns over sensitivity and specificity require further validation studies to confirm the test’s reliability and accuracy.
Finally, Signatera detects ctDNA for personalized monitoring of cancer patients post-treatment. The personalized approach targets specific mutations or alterations unique to each patient’s tumor, providing exceptional sensitivity in detecting low ctDNA levels. However, ctDNA detection bears limitations related to tumor location, heterogeneity, and shedding dynamics, leading to challenges in accurately capturing all MRD cases and potentially resulting in false negatives and missed opportunities for early intervention.
Natera Outperforms Competitors
When comparing Natera’s products with those of its competitors, it becomes evident that Natera has developed cutting-edge and robust technology that addresses various critical needs in the healthcare industry.
A primary competitor in the NIPT landscape is Illumina (ILMN), which developed the Verifi Prenatal Test. This test also relies on cell-free DNA analysis to detect common chromosomal conditions, including Down syndrome, Edwards syndrome, and Patau syndrome. Although the Verifi test is highly sensitive and specific, Panorama’s unique capability to differentiate between the DNA of the pregnant individual and the baby provides a crucial advantage. This ability enables Panorama to screen for triploidy, which can have severe consequences for both the pregnancy and the pregnant individual’s health, thus addressing a clinical gap not covered by traditional NIPTs like Verifi.
In the transplant monitoring domain, competitors like CareDx (CDNA) have developed noninvasive blood tests such as AlloSure for kidney transplant monitoring. While AlloSure also uses dd-cfDNA analysis for identifying rejection, Prospera’s use of 13,000 pan-ethnic SNPs and advanced bioinformatics enables the detection of both AMR and ACR. This comprehensive approach allows for more precise rejection risk assessment and improved patient outcomes, making Prospera an attractive alternative to AlloSure for heart transplant surveillance.
Guardant Health’s (GH) Guardant360 is a competitor of Signatera in the liquid biopsy space, designed for comprehensive genomic profiling of solid tumors for targeted cancer therapy selection. Although Guardant360 provides valuable insights into tumor biology and suitable therapeutics, it is not explicitly designed for MRD monitoring. Signatera’s personalized and sensitive approach-tailored to each patient’s tumor mutational landscape-makes it superior for detecting and tracking MRD, offering a substantially greater chance of identifying cancer recurrence at its earliest stages.
Conclusion
Natera’s impressive performance and dedication to innovation have made them a dominant force in the genetic testing arena. The company’s pioneering products-Panorama, Prospera, and Signatera-show its remarkable ability to meet the diverse needs of patients and healthcare professionals. With a solid foundation of cutting-edge technology and intellectual property, it is clear that Natera is set to radically redefine the realms of prenatal care, organ transplant monitoring, and cancer surveillance.
As a result, investors seeking to capitalize on the dynamic potential of the rapidly evolving healthcare sector will likely find Natera to be an attractive and rewarding investment opportunity. The company’s strategic positioning and relentless drive for growth, combined with an impressive track record of financial and operational success, make Natera uniquely poised to lead the charge and deliver substantial value for shareholders in the years to come.
Read the full article here





